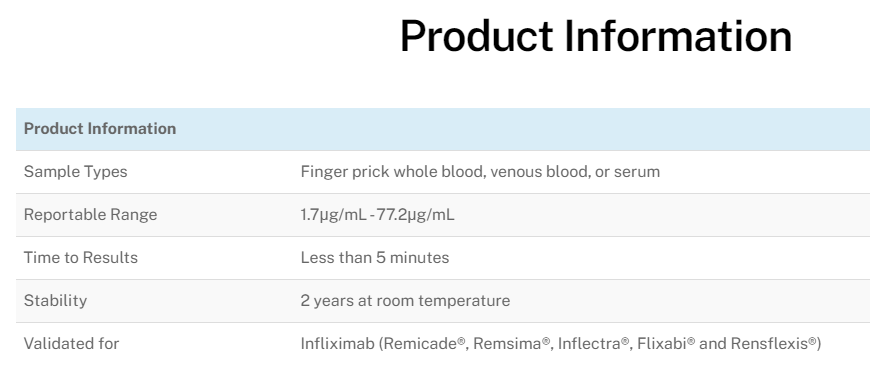
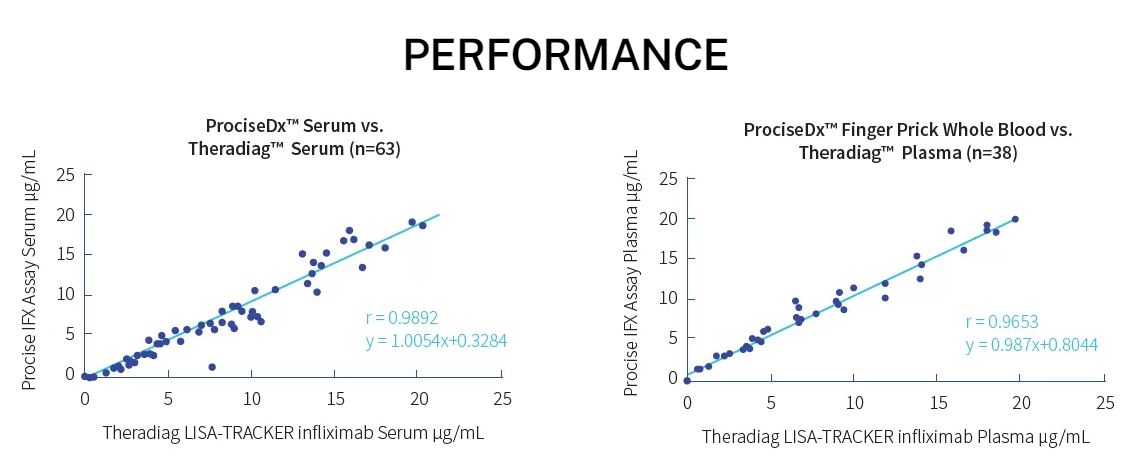
Over the past two decades great improvements in therapy of chronic inflammatory diseases have been made. The rise of TNF biologics like infliximab has been a great step forward to ameliorating disease course and keep inflammations at remission levels for prolonged periods of time. Therapeutic Drug Monitoring (TDM) of biotherapies is a well recognized tool allowing a better usage of biotherapies by making evidence-based treatment decisions. However, all the benefits of TDM for infliximab are not available on a true point-of-care platform. Current limitations in testing force dose adjustments to come weeks after trough level testing. Procise IFX allows immediate decisions on drug dose adjustments at the time of infusion in only 5 minutes with finger prick blood by virtually any level laboratory technician.

EASY. IMMEDIATE. ACCURATE. The Point-of-Care platform with unprecedented capabilities.
Therapeutic Drug Monitoring (TDM) of antitumor necrosis factor (TNF) such as Adalimumab has increased over time as doctors improve their treatment of IBD. Even if the medication is administered subcutaneously by the patient, there is a need to check trough levels before medication to ensure proper levels are maintained. However, obtaining results from previously available testing methods take days or even weeks. Procise ADL allows for true point-of-care testing of Adalimumab levels in under 5 minutes with finger stick blood. So during a standard visit with an IBD patient, a physician or technician can check their levels and adjust medication accordingly. During their very next injection, not weeks or months later.
C-Reactive Protein (CRP) is a marker of inflammation and increases with active disease. It is produced by the liver during episodes of acute inflammation or infection and useful for monitoring your IBD. Empower your office with the ability to test patients for flares ups during their visit. Help them with answers and actions today instead of a lab order and more waiting. In just 2 minutes, ProciseDx will provide lab quality quantitative results for CRP from a finger prick blood sample. Simply collect a finger prick blood sample, add the sample and a buffer bulb, then run the test on ProciseDx. Any extra samples preparation, wait times or manual steps are unacceptable in point-of-care testing.

Fecal calprotectin is commonly used to help in differentiation between irritable bowel syndrome (IBS) and inflammatory bowel diseases (IBD). It is a sensitive and stable biomarker which is not affected by medication or dietary supplements. Quantitative results can give a clear answer for new diagnosis, as well as valuable tracking information for ongoing treatment. Additionally, fecal calprotectin is an excellent predictor of relapse in patients with IBD. Clinicians depend on it to adapt their patient’s treatment and to ease the severity of potential relapse. FCP can add information and value to an endoscopy, or in some cases eliminate the need for invasive procedures. With ProciseDx, this test can be done in under 5 minutes during a patient visit.

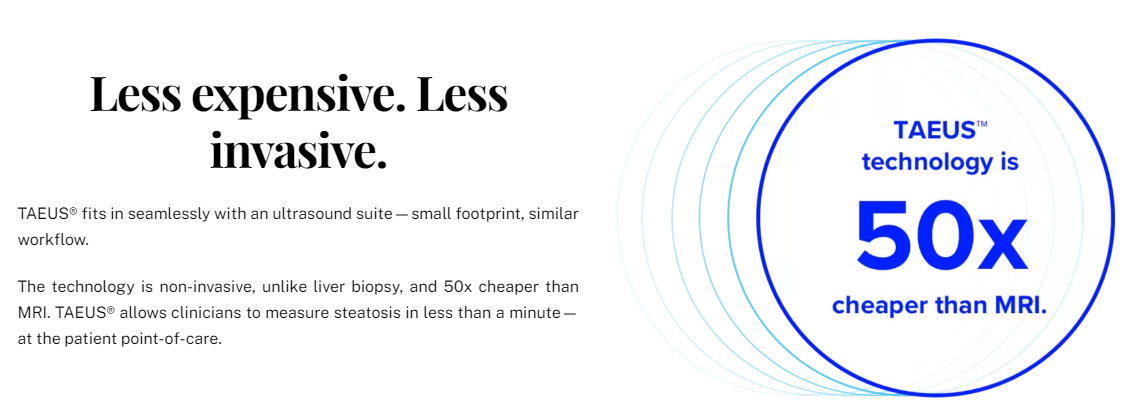
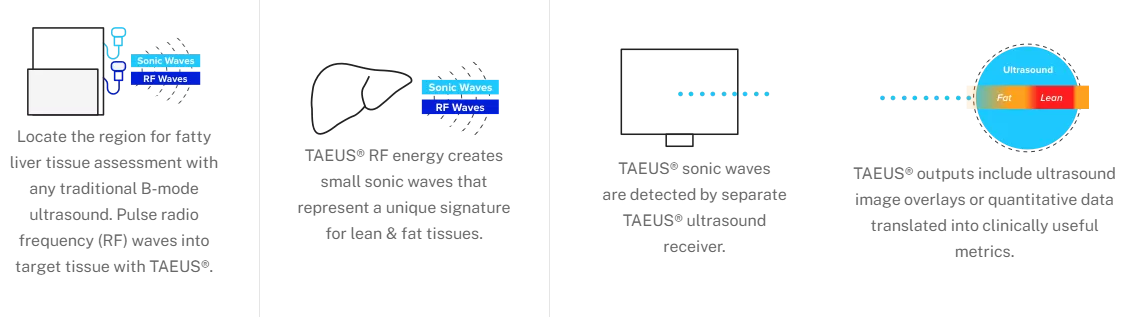
We’re changing the way clinicians measure fat in the liver, making it easier, less expensive, and more accessible for everyone. Thermo-Acoustic Enhanced Ultrasound® (TAEUS®) combines radio frequency and ultrasound waves to measure fat in the liver in a ground-breaking way. It is CE-marked and commercially available in Europe, and pairs seamlessly with any B-mode ultrasound machine.